Results of SOLAR-1
SOLAR-1 is a double-blind, placebo‑controlled, multicenter phase 3 study in men and postmenopausal women with HR+/HER2- advanced or metastatic breast cancer with or without a PIK3CA mutation whose disease had progressed or recurred on or after AI-based treatment (N=572). In the PIK3CA mutation cohort (n=341), patients were randomized 1:1 to receive PIQRAY 300-mg tablets orally once daily + fulvestrant 500 mg IM (n=169)* or placebo + fulvestrant 500 mg IM (n=172).* The primary endpoint was PFS in patients with a PIK3CA mutation by investigator assessment per RECIST v1.1. See full study design.
*Fulvestrant given on day 1 and day 15 of the first 28-day cycle, then on day 1 of subsequent 28-day cycles.
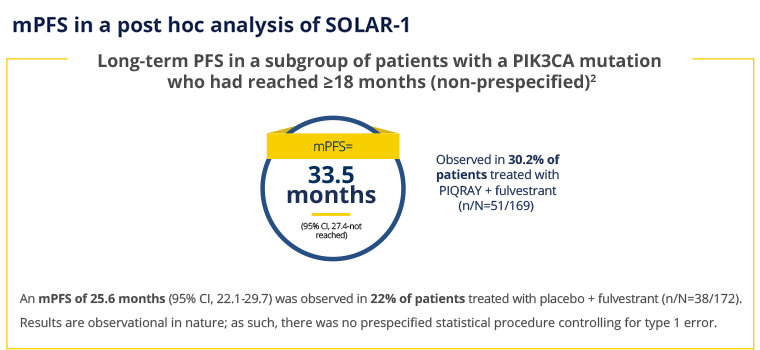
PIQRAY + fulvestrant nearly doubled mPFS in patients with a PIK3CA driver mutation1
At the prespecified final OS analysis, there was no significant difference in OS between the PIQRAY plus fulvestrant arm and the placebo plus fulvestrant arm (hazard ratio [HR] = 0.86, 95% CI: 0.64, 1.15).1
Highlights of Important Safety Information
PIQRAY is contraindicated in patients with severe hypersensitivity to it or any of its components.
Serious adverse reactions include severe hypersensitivity, severe cutaneous adverse reactions, hyperglycemia, pneumonitis, diarrhea or colitis, and embryo-fetal toxicity.
Most common adverse reactions including laboratory abnormalities (all grades, incidence ≥20%) were glucose increased, creatinine increased, diarrhea, rash, lymphocyte count decreased, gamma-glutamyl transferase increased, nausea, alanine aminotransferase increased, fatigue, hemoglobin decreased, lipase increased, decreased appetite, stomatitis, vomiting, weight decreased, calcium decreased, glucose decreased, activated partial thromboplastin time prolonged, and alopecia.
Please see additional Important Safety Information below.
Please see full Prescribing Information.
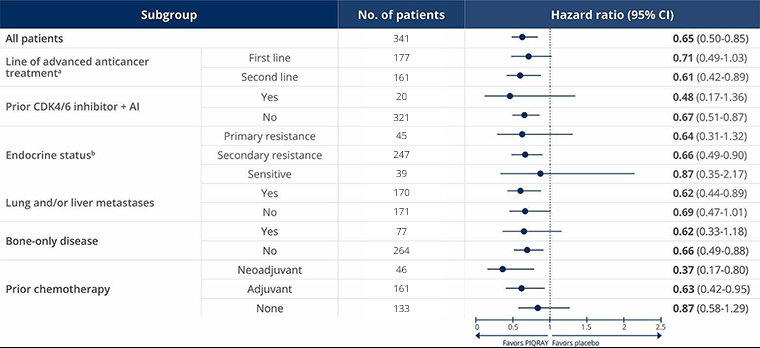
Consistent PFS was seen across subgroups, including those with prior CDK4/6 inhibitor + AI or primary/secondary endocrine resistance3
PFS in select patient subgroups with a PIK3CA mutation3
The data are from prespecified subgroup analyses of the primary endpoint (mPFS) in SOLAR-1 and are not powered to detect statistical significance.
aIn the SOLAR-1 study, first line was defined as patients whose disease progressed ≤1 year after (neo)adjuvant ET or whose disease progressed >1 year after (neo)adjuvant ET, and who did not receive prior treatment for aBC. Second line was defined as patients whose disease progressed >1 year after (neo)adjuvant ET and while on or after one line of ET for aBC or patients with newly diagnosed aBC whose disease progressed while on or after one line of ET.
bIn the SOLAR-1 study, primary endocrine resistance was defined as relapse within 24 months on adjuvant ET or progression within 6 months on ET for advanced disease. Secondary endocrine resistance was defined as relapse after 24 months on adjuvant ET, relapse within 12 months of the end of adjuvant ET, or progression after 6 months on ET for advanced disease. Endocrine sensitive was defined as relapse ≥12 months after completion of ET in the adjuvant setting.
aBC, advanced breast cancer; AI, aromatase inhibitor; CDK, cyclin D–dependent kinase; ET, endocrine therapy; IM, intramuscularly; mPFS, median progression-free survival; OS, overall survival; PFS, progression-free survival.
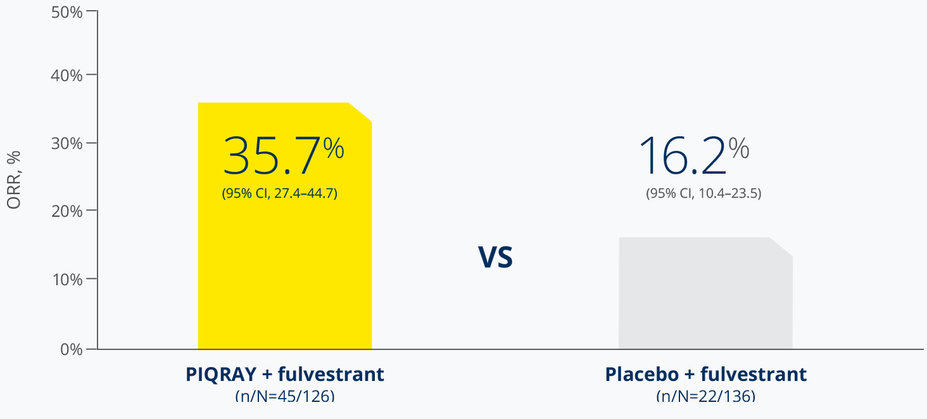
PIQRAY + fulvestrant more than doubled the response rate in patients with a PIK3CA driver mutation1
Overall response rate (ORR) in patients who had measurable disease1
ORR was defined as the percentage of subjects with confirmed complete response or partial response. Measurable disease was defined as the presence of at least one measurable nodal or non-nodal lesion as per RECIST v1.1 criteria.
Highlights of Important Safety Information
PIQRAY is contraindicated in patients with severe hypersensitivity to it or any of its components.
Serious adverse reactions include severe hypersensitivity, severe cutaneous adverse reactions, hyperglycemia, pneumonitis, diarrhea or colitis, and embryo-fetal toxicity.
Most common adverse reactions including laboratory abnormalities (all grades, incidence ≥20%) were glucose increased, creatinine increased, diarrhea, rash, lymphocyte count decreased, gamma-glutamyl transferase increased, nausea, alanine aminotransferase increased, fatigue, hemoglobin decreased, lipase increased, decreased appetite, stomatitis, vomiting, weight decreased, calcium decreased, glucose decreased, activated partial thromboplastin time prolonged, and alopecia.
Please see additional Important Safety Information below.
Please see full Prescribing Information.
AI, aromatase inhibitor; IM, intramuscularly.