SOLAR-1 Study Design
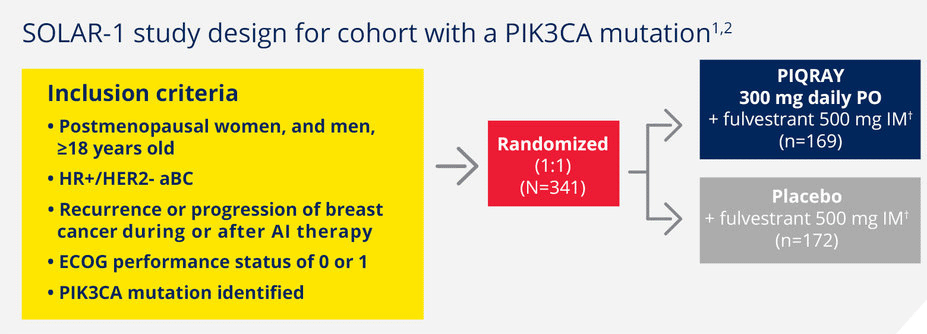
SOLAR-1 was the first phase 3 trial leading to an approval specifically for aBC patients with a PIK3CA mutation1
Randomized, double-blind, prospective, placebo-controlled trial in HR+/HER2- aBC1
A total of 572 patients were enrolled in the trial, including 341 patients with a PIK3CA mutation.1*
Patients were stratified by1:
Presence of liver and/or lung metastases
Prior CDK4/6 inhibitor treatment
231 of these patients did not have a PIK3CA mutation and were included as a separate cohort as a proof of concept, but the proof of concept criteria were not met for this cohort. No PFS benefit was observed in patients whose tumors did not have a PIK3CA tissue mutation (HR=0.85; 95% CI, 0.58-1.25).1
In the SOLAR-1 trial, patients with controlled type 2 diabetes and prediabetes were included if they had a fasting plasma glucose (FPG) of ≤140 mg/dL (7.7 mmol/L) and glycosylated hemoglobin (HbA1c) ≤6.4% (both criteria had to be met).1
aBC, advanced breast cancer; AI, aromatase inhibitor; CDK, cyclin D–dependent kinase; ECOG, Eastern Cooperative Oncology Group; IM, intramuscularly; PO, orally.
*A total of 572 patients were enrolled in the SOLAR-1 trial, but 571 patients were included in safety assessments.
†Fulvestrant given on day 1 and day 15 of the first 28-day cycle, then on day 1 of subsequent 28-day cycles.
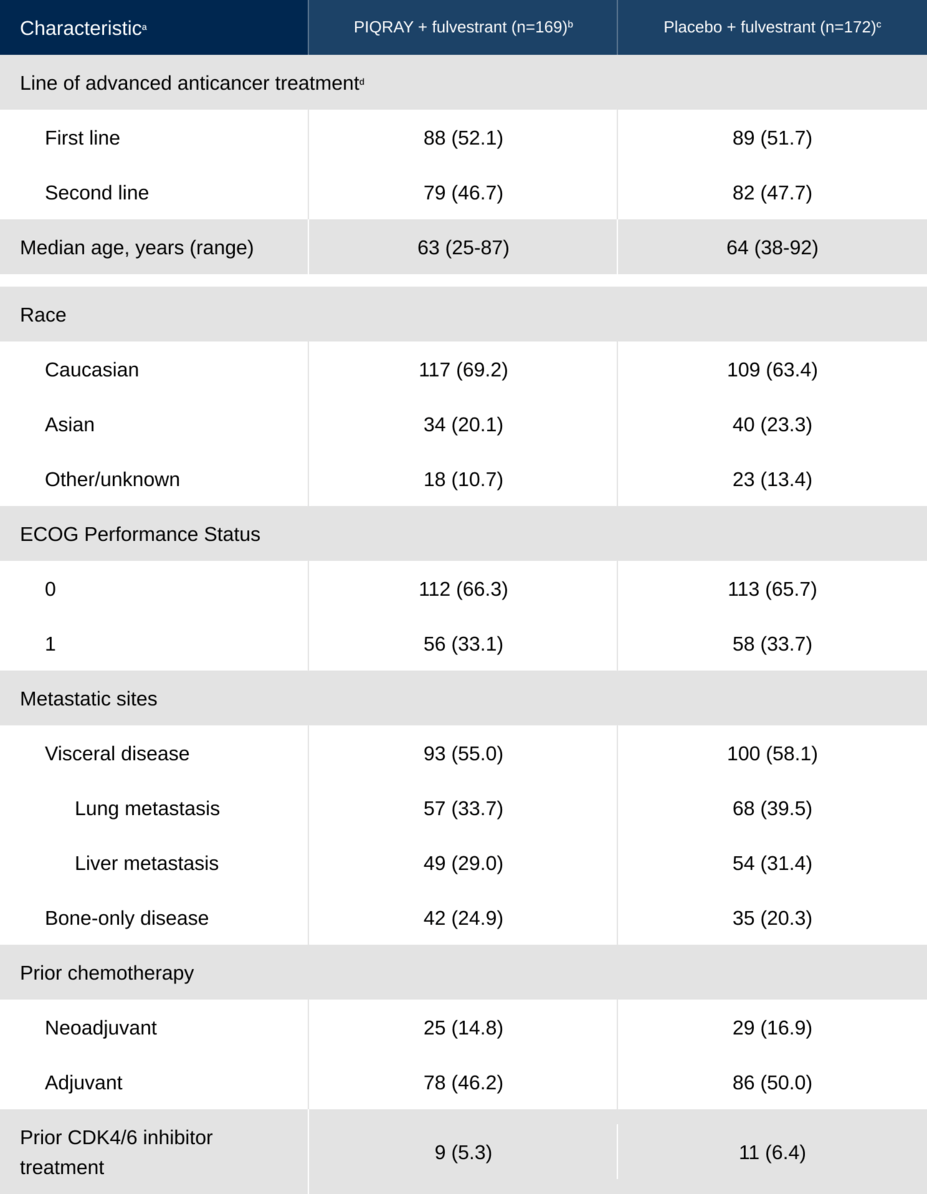
Patients with a broad range of characteristics were represented
Baseline characteristics of patients in SOLAR-1 with a PIK3CA mutation2,3
aCharacteristics are given as n (%) unless otherwise stated.
bOne man was enrolled in the PIQRAY + fulvestrant arm. All other study participants were postmenopausal women.
cOne patient randomized to placebo was not treated.
dIn the SOLAR-1 study, first line was defined as patients whose disease progressed ≤1 year after (neo)adjuvant endocrine therapy (ET) or whose disease progressed >1 year after (neo)adjuvant ET, and who did not receive prior treatment for aBC. Second line was defined as patients whose disease progressed >1 year after (neo)adjuvant ET and while on or after one line of ET for aBC or patients with newly diagnosed aBC whose disease progressed while on or after one line of ET.