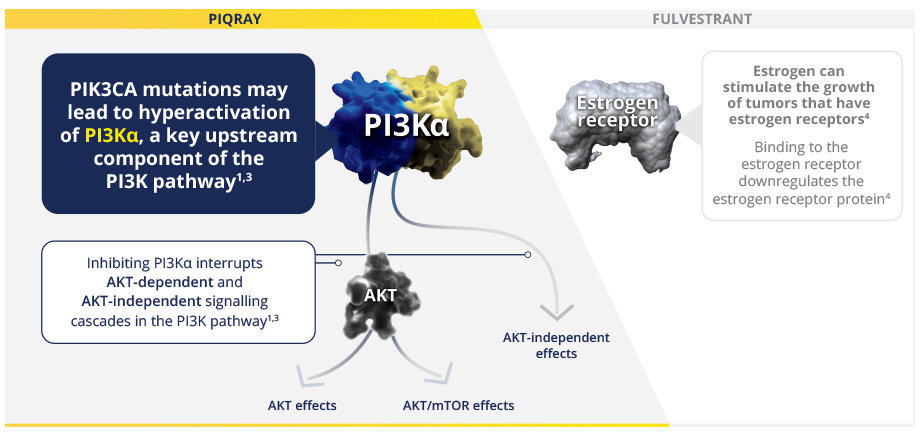
PIQRAY Directly Targets the PI3K Pathway, Which May Help Overcome Endocrine Resistance1
PI3K inhibition with PIQRAY has been shown to induce an increase in estrogen receptor (ER) transcription in breast cancer cells1
PIQRAY inhibits the alpha isoform of PI3K 50 times more potently than other PI3K isoforms (β, γ, δ)2
PIQRAY is a nonchemotherapeutic α-selective PI3K inhibitor that works synergistically with fulvestrant across both the PI3K and ER pathways, respectively1
Based on in vitro/in vivo studies. Preclinical activity does not necessarily correlate with clinical outcomes.
PI3Kα, α isoform of phosphatidylinositol-3-kinase.
PIK3CA is a common driver mutation in HR+/HER2- aBC with effects that may be addressed with a targeted approach1,5