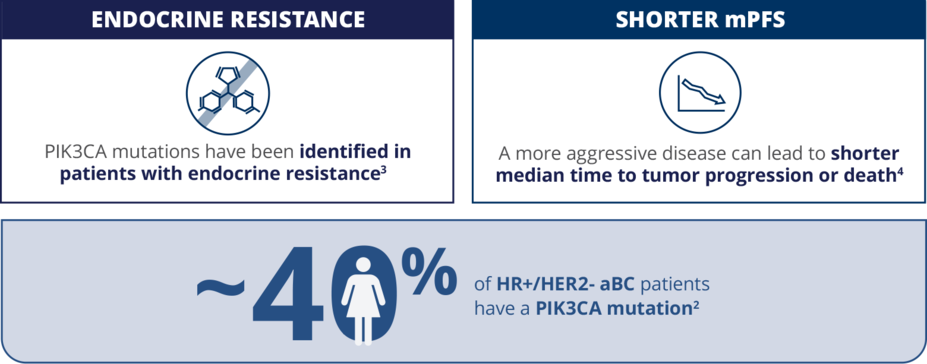
Patients with a PIK3CA Mutation Face a Poor Prognosis1
PIK3CA is the most commonly mutated gene in HR+/HER2- aBC2
Patients with a PIK3CA mutation can have:
mPFS, median progression-free survival.