Test Patients for PIK3CA Mutations at HR+/HER2- MBC Diagnosis to Be Ready to Treat at Progression*
*Following progression on or after an endocrine-based regimen.
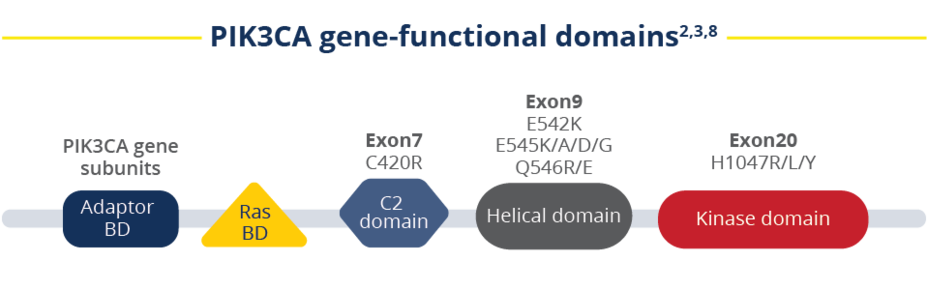
As the most common mutation in HR+/HER2- aBC, it’s important to test for PIK3CA mutations early on during the initial MBC workup.2 With 3 FDA-approved testing modalities, you have multiple options for learning their PIK3CA mutation status.2,3
As patients with PIK3CA mutations face a poor prognosis, it's important to test their status.4
Learn more about the effects of PIK3CA mutations in MBC →
The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way.
*Following progression on or after an endocrine-based regimen.

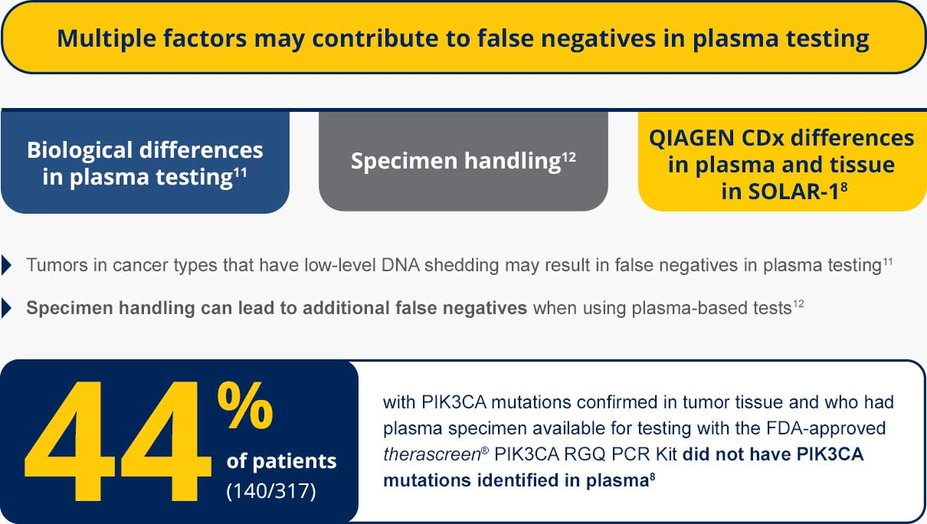
There are 2 FDA-approved PIK3CA mutation CDx tests available to eligible patients2,3
Select from the options below to learn more about each test.
Foundation Medicine's FoundationOne®CDx and FoundationOne®Liquid CDx are comprehensive genomic profiling (CGP), next-generation sequencing (NGS) multi-gene tests, which include PIK3CA, and provide tissue and plasma testing, respectively.2,3
Multi-gene NGS test from Foundation Medicine
FoundationOne®CDx tissue-based
FoundationOne®CDx is a comprehensive genomic profiling (CGP) next-generation sequencing (NGS)-based multi-gene in vitro diagnostic test. FoundationOne®CDx can detect PIK3CA mutations in tissue and is a companion diagnostic for alpelisib.2
SEE TECHNICAL AND SAFETY INFORMATION
FoundationOne®CDx and FoundationOne®Liquid CDx are covered by Original Medicare and Medicare Advantage for qualifying beneficiaries
Results can be found on page 1 of the sample report.
Foundation Medicine offers in-home blood draw with mobile phlebotomy through its partner, ExamOne®, to support broader access to FoundationOne®Liquid CDx, at no additional cost.
Foundation Medicine generally expects to provide results in 12 days or less from specimen receipt.
Multi-gene NGS test from Foundation Medicine
FoundationOne®Liquid CDx plasma-based
FoundationOne®Liquid CDx is a comprehensive genomic profiling (CGP) next-generation sequencing (NGS)-based multi-gene in vitro diagnostic test. FoundationOne®Liquid CDx can detect PIK3CA mutations in blood and is a companion diagnostic for alpelisib. Foundation Medicine offers the option to automatically reflex between tissue and liquid sample types.3
TECHNICAL AND SAFETY INFORMATION
FoundationOne®CDx and FoundationOne®Liquid CDx are covered by Original Medicare and Medicare Advantage for qualifying beneficiaries12
Results can be found on page 1 of the sample report.
Foundation Medicine offers in-home blood draw with mobile phlebotomy through its partner, ExamOne®, to support broader access to FoundationOne®Liquid CDx, at no additional cost.
Novartis is not responsible for any such third-party content that may be accessed via the above provided resources. Novartis does not endorse the content contained in these sites, nor the organizations publishing those sites, and hereby disclaims any responsibility for such content.
Foundation Medicine generally expects to provide results in 12 days or less from specimen receipt.